Due to the results of this study, an application for an additional indication for icosapent ethyl to reduce the risk of major CVD events was submitted to the FDA in March 2019 and is pending. Currently, this agent is approved only to treat patients with TG levels ≥500 mg/ dL to prevent acute pancreatitis.23 Although not yet FDA approved for the CV indication, recent guidelines have been modified to recommend this agent in certain patients. The American Diabetes Association (ADA) issued an update in March 2019 to its standards of care, advising that icosapent ethyl be considered to reduce CV risk in patients with diabetes and ASCVD or with other cardiac risk factors who are on a statin and have controlled LDL-C, but elevated TG levels.24
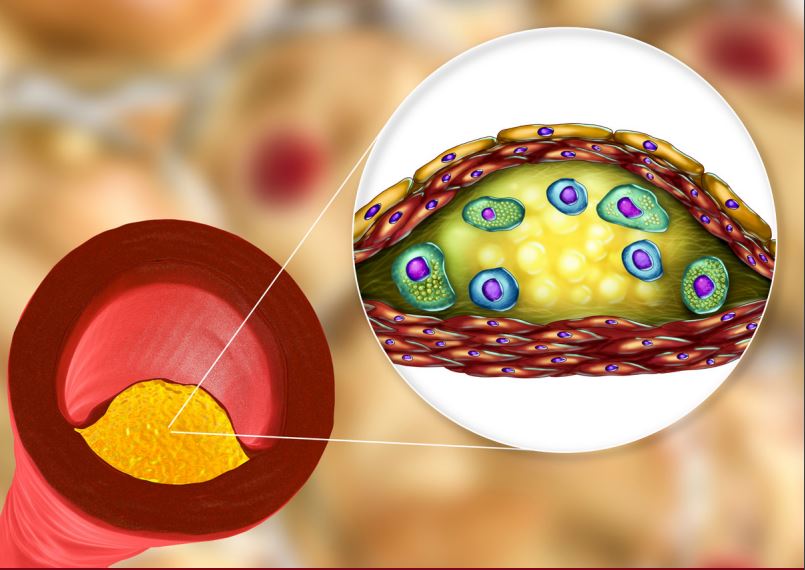
Recent results with icosapent ethyl, as demonstrated by the REDUCE-IT trial, have shown that it can significantly reduce atherosclerotic events in high-risk hypertriglyceridemic patients with clinical ASCVD or type 2 diabetes and additional markers of increased risk. As more evidence becomes available, it is expected that icosapent ethyl will play an important role in primary and secondary prevention in high-risk patients.