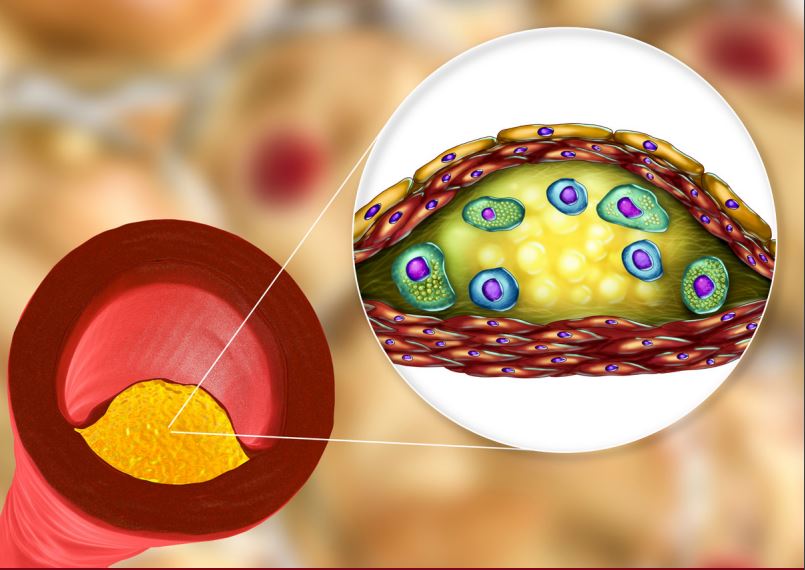
Omega-3 fatty acids have been shown to reduce TG levels by 22-33% in statin-treated patients with high baseline levels of TG (25680 mg/dL) in short-term studies.17,18 Recent meta-analyses did not provide support for the role of OM3FA supplements in CVD risk, however, different formulations were used in these studies, including not completely pure OM3FA, which makes their interpretation challenging.4,19,20 To date, the one study that used a pure OM3FA agent (1.8 mg EPA) in addition to low-dose statin, JELIS, showed that EPA was associated with a 19% reduction in major coronary events.21 The ASCEND trial, which assessed the efficacy and safety of daily supplementation with OM3FA (1 g EPA + DHA) in preventing CV events (a combination of non-fatal MI, non-fatal stroke or TIA, and vascular death) in patients with diabetes and no prior cardiovascular disease, showed that OM3FA supplementation did not prevent major adverse CV events.22 However, these results of the ASCEND trial could be due to the dose of OM3FA used (1g), which has not shown positive results in previous trials, and authors suggest that higher doses (2-4 g) may yield more benefits.4,22
Recently, the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT), which used higher doses of a highly purified ester of EPA, icosapent ethyl, evaluated the effects of this intervention in preventing cardiovascular events in high-risk patients with established ASCVD or diabetes plus at least an additional risk factor.5 A total of 8179 patients, who had fasting triglyceride levels of 135-499 mg/dL and an LDL-C of 41-100 mg/dL while on background statin therapy were enrolled and randomized to receive 2g of icosapent ethyl twice daily (4g total daily dose) or placebo.5 In this trial, patients treated with icosapent ethyl had a 25% risk reduction in the occurrence of major adverse CV events (composite of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina requiring hospitalization) after a median follow-up of 4.9 years, which was significant compared to placebo.5 This included a statistically significant 20% reduction in CV death, as well as statistically significant relative risk (RR) reductions for other prespecified individual endpoints, including myocardial infarction (31% RR), stroke (28% RR), hospitalization for unstable angina (32% RR), and urgent or emergent coronary revascularization (35% RR) compared to placebo.2 Furthermore, the benefits were consistent among several prespecified subgroups, including in patients with normal TG levels (<150 mg/dL), which represented 10.3% of the study population.2 Thus, it is unlikely that the benefits observed with icosapent ethyl in the REDUCE-IT trial are merely a function of baseline TG levels, since the risk reduction in the primary and key secondary endpoints was relatively the same in those with baseline TG ≥200 mg/dL and those with what is TG values of ≥150 mg/dL, and these results are re-defining of what we perceive to be “normal” TG levels.2,23 The specific mechanisms of action that lead to these benefits with icosapent ethyl are an ongoing area of investigation.23 In this trial, icosapent ethyl was generally well tolerated; with a trend toward more bleeding-related disorders which did not reach statistical significance, and increased hospitalization rates for atrial fibrillation with icosapent ethyl compared to placebo.5 A recent sub-analysis of the REDUCE-IT trial showed that in addition to the 25% reduction in first ischemic events, icosapent ethyl was associated with a 32% reduction in second events, a 31% reduction in third events, and a 48% reduction in fourth of subsequent events, with total events being reduced by 30% compared to placebo.6