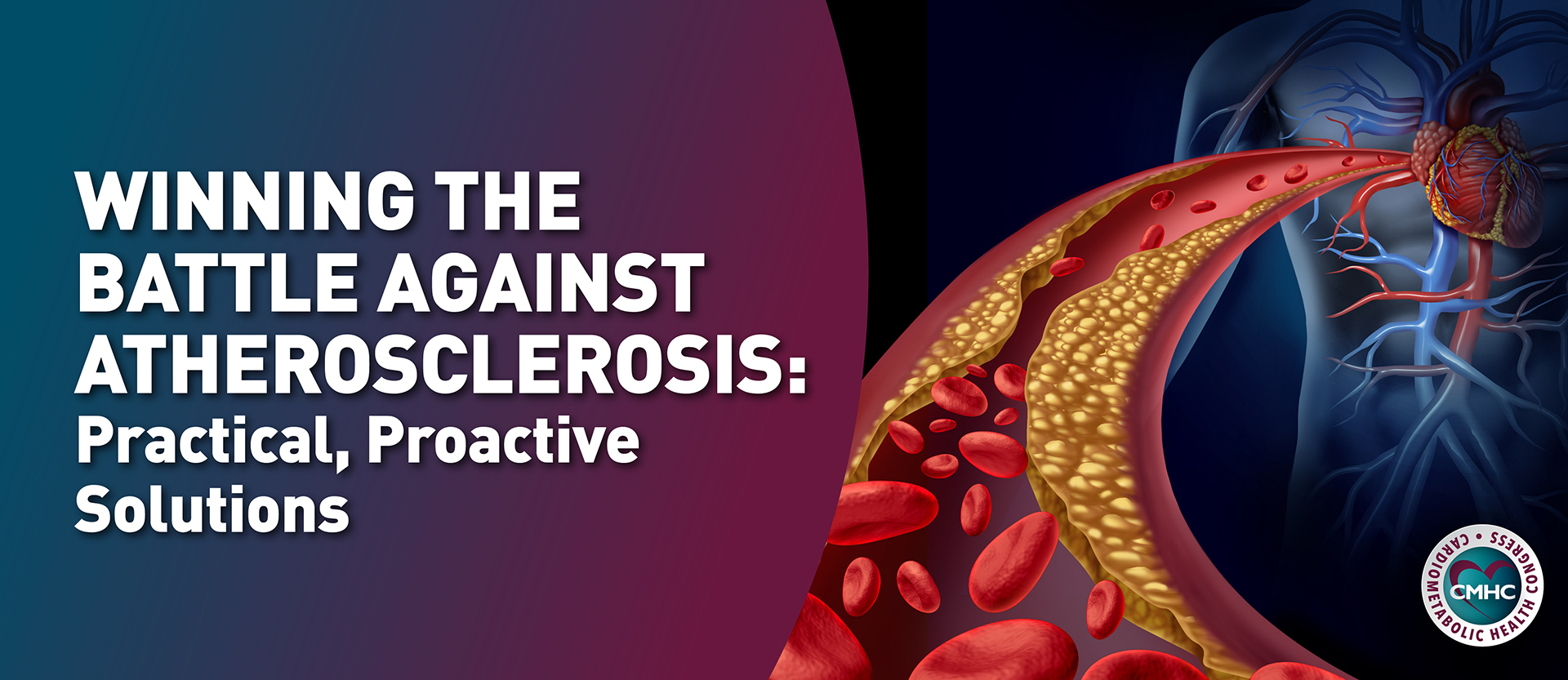
Over the last 20 years, there has been an increase in the incidence of pediatric venous thromboembolism (VTE) – which includes venous thrombosis and pulmonary embolism (PE) – especially in hospitalized pediatric patients. In fact, the incidence of VTE in hospitalized children increased by 130% from 2008 to 2019, with 106 VTE cases per 10,000 admissions. This uptick is believed to be a result of an increase in awareness and better detection of VTE in children, and also attributed to an improved treatment and survival of children with life-threatening or chronic medical conditions.
Pediatric VTE is a serious medical condition, and it can cause various harms including death, PE, paradoxical emboli and stroke, organ dysfunction, infection, post-thrombotic syndrome (PTS), loss of venous access, and pain. Hospital-acquired VTE is associated with an increase in cost and longer hospital stays.
While likely an underestimate, pediatric VTE has a current mortality rate of 2.2%; anticoagulation treatment options for children have been historically scarce with few clinical trials done in this population group and many agents not approved for use in children. The physiological differences between pediatric and adult patients and the rapid growth and metabolic changes characteristic of childhood make it challenging to determine the correct anticoagulation dose, and the absence of child-friendly formulations makes accurate dosing difficult.
Risk factors and clinical manifestations
Pediatric VTE has a bimodal age distribution, being more common in neonates, as a complication of life sustaining therapies provided to premature and critically ill infants, and adolescents, reflecting the development of adult VTE risk factors. The presence of a central venous access device (CVAD) is the most common precipitating factor for the occurrence of VTE in children. However, other risk factors include congenital heart disease, trauma and surgery, infection (local or systemic), malignancy, prematurity, oral contraceptives, immobilization, the presence of antiphospholipid antibodies, and inherited thrombophilia. Most pediatric VTE patients have multiple risk factors.
Children who develop VTE due to an identifiable underlying condition or risk factor (provoked VTE) with no prior history of VTE, a VTE that is not severe or life threatening, a transient provoking risk factor that resolves within six weeks, and a thrombus that resolves or is nonocclusive within six weeks, are considered at a low risk of experiencing VTE recurrence and other complications. Recently published results from a clinical trial support treating this group differently from other pediatric VTE populations.
Although it is rare, some children develop VTE without an underlying risk factor, called unprovoked VTE. Testing for inherited thrombophilia should be considered for these patients. There are several genetic risk factors that predispose individuals to developing VTE. The risk of VTE is highest in individuals with antithrombin, protein C or protein S deficiency, and those with homozygous factor V Leiden or prothrombin G20210A mutations.
As with adult patients, the clinical manifestations of pediatric VTE vary, depending on the location of the thrombus and degree of vessel occlusion. A central venous catheter (CVC)-related VTE is most often asymptomatic but can also present as repeated loss of CVC patency, CVC-related sepsis, swelling and discoloration of the related limb, facial swelling and other signs of superior vena cava syndrome, prominent collateral circulation in the skin over the chest, back, neck, and face, pulmonary embolism, and chylothorax. Deep vein thrombosis is more likely to occur in the lower extremities and the clinical manifestations are similar to those seen in adult patients with VTE, as well as for other venous thrombosis (renal and portal vein thrombosis, and cerebral venous thrombosis) and for PE.
Treatment guidelines
The primary goal of treating VTE is to halt the progression of the clot and aid in its resolution, the second goal is to prevent embolization of the thrombus, and the third goal is to prevent VTE recurrence, all while minimizing long-term complications. Conventional anticoagulants do not cause clot breakdown. Instead, they only prevent clot expansion and rely on the endogenous fibrinolytic system to dissolve the thrombus.
Treatment options for pediatric VTE include unfractionated heparin (UFH), low molecular weight heparin (LMWH), warfarin, fondaparinux, and direct thrombin inhibitors (DTIs). Until last year, direct oral anticoagulants (DOACs) were not approved for the treatment of pediatric patients and, therefore, were not included in clinical guidelines for pediatric VTE. In addition to anticoagulation agents, VTE can also be managed with thrombolytic agents. When deciding on a treatment plan, the risk of complications from the thrombus need to be balanced against the risk of treatment.
Initial treatment consists of a parenteral agent (UFH or LMWH) for the first few days, followed by either parenteral or oral therapy (LMWH, warfarin, or a DOAC). Recently published results from a clinical trial showed that for low-risk patients, therapy can be given for 6 weeks instead of the standard 3 months.10 For all other patients, anticoagulation therapy should be given for at least 3 months. Unprovoked VTE is treated with anticoagulation therapy for 6-12 months or indefinitely for children with recurrent unprovoked VTE.
Treatment landscape
Until last year, warfarin, a vitamin K antagonist, was the only oral agent used to treat pediatric VTE. While its use in older children is feasible, it has various disadvantages that makes its use complicated, including: the need for frequent monitoring; numerous drug interactions; being affected by dietary intake of vitamin K; and a narrow therapeutic range. Also, since warfarin tablets cannot be compounded into a liquid formulation, administration in young children is difficult. In neonates, this is further complicated by the physiologically low levels of vitamin K-dependent clotting factors and the low vitamin K content of breast milk. The most important complication to monitor is bleeding, which can be serious and life-threatening. However, despite these disadvantages, warfarin may be preferred by patients requiring long-term therapy because it is administered orally.Two new agents were approved by the U.S. Food and Drug Administration (FDA) for the treatment of pediatric VTE in 2021. This year, results from a clinical trial showed that the treatment period for children with low-risk VTE can be significantly shortened. These updates represent a significant change to the management of pediatric VTE and there is a need to inform health care professionals that are likely to treat these patients.
- Anticoagulation agents. Initial VTE management of pediatric patients is usually done with heparins, including UFH and LMWHs. The first-line therapy for hospitalized pediatric patients with VTE is UFH, which is administered as a continuous intravenous infusion. UFH is also used for primary prophylaxis of VTE for individuals with congenital heart disease undergoing certain procedures or surgical interventions. LMWHs (including enoxaparin and dalteparin) are also widely used in pediatrics as they have certain advantages over UFH including a greater and more predictable
 bioavailability, longer duration of anticoagulation effect, and reduced complication rates of heparin-induced thrombocytopenia (HIT) and osteoporosis. The main disadvantage of LMWHs is that the administration is done by twice daily subcutaneous injections. Fondaparinux, a synthetic pentasaccharide, is another anticoagulant used to treat pediatric VTE. In addition to having all of the advantages that LMWHs have, it is administered once-daily and it has no risk for HIT or osteoporosis, making it an attractive alternative. DTIs, more targeted short-acting agents including bivalirudin and argatroban, are also administered by continuous intravenous infusion. DTIs are used primarily in pediatric cases of HIT, which is a rare but potentially life-threatening condition. The pharmacokinetics of DTIs are also more predictable than those of UFH.
bioavailability, longer duration of anticoagulation effect, and reduced complication rates of heparin-induced thrombocytopenia (HIT) and osteoporosis. The main disadvantage of LMWHs is that the administration is done by twice daily subcutaneous injections. Fondaparinux, a synthetic pentasaccharide, is another anticoagulant used to treat pediatric VTE. In addition to having all of the advantages that LMWHs have, it is administered once-daily and it has no risk for HIT or osteoporosis, making it an attractive alternative. DTIs, more targeted short-acting agents including bivalirudin and argatroban, are also administered by continuous intravenous infusion. DTIs are used primarily in pediatric cases of HIT, which is a rare but potentially life-threatening condition. The pharmacokinetics of DTIs are also more predictable than those of UFH. - DOACs. Recently, the FDA approved the use of DOACs for the treatment and prevention of VTE in certain pediatric populations for the first time. DOACs for the treatment of pediatric VTE and how they fit into the existing clinical guidelines. The drawbacks of traditional anticoagulants described above highlight the need for new medications for children with targeted mechanisms of action, oral formulations (including liquid), few drug and food interactions, and requiring little or no therapeutic drug monitoring. This need prompted the design of clinical trials to test the safety and efficacy of DOACs, which fit all these requirements, in pediatric populations. DOACs are widely used in adult populations and have many advantages compared with warfarin such as a rapid onset/offset of action, absence of food interactions, limited hepatic metabolism, and few strong drug interactions, greater convenience, lower risk of intracranial hemorrhage and lower potential risk of bleeding complications, and may be more cost-effective. On the other hand, they also have some disadvantages including being counterindicated for patients with severe chronic kidney disease, limited availability of assays for measuring drug levels and no validated monitoring strategies, potential for over-use, higher drug acquisition costs, short half-life, and no specific antidote.
- Factor Xa inhibitors. The first direct factor Xa inhibitor approved for adult patients was rivaroxaban, an active side-directed, reversible inhibitor that prolongs the prothrombin time and the activated partial thromboplastin time. In 2021, the European Medicines Agency and the Food and Drug Administration also approved it to treat and prevent VTE in certain pediatric patients. The EINSTEIN-Jr study (NCT02234843; completed in 2020) was the first completed pediatric, phase 3 DOAC clinical trial, and found that rivaroxaban was equivalent to standard therapy in lowering the risk of recurrent VTE and clinically relevant bleeding. Furthermore, treatment with rivaroxaban resulted in a greater reduction of thrombus mass, with a favorable safety profile. Similarly, the UNIVERSE study (NCT02846532) showed that rivaroxaban had a similar safety profile to standard of care therapy when given prophylactically to patients who had undergone the Fontane procedure. Another direct, reversible inhibitor of factor Xa is apixaban, which inhibits free and clot-bound factor Xa, prothrombinase activity, and platelet aggregation induced by thrombin. A series of clinical trials in pediatric patients have been completed recently (NCT01707394, NCT02369653, NCT02981472), but the results have not been published yet. A clinical trial (NCT02464969) is currently evaluating its safety and efficacy in pediatric patients requiring anticoagulation for the treatment of VTE. Finally, edoxaban inhibits free factor Xa, which leads to decreased thrombin generation and indirect inhibition of platelet aggregation. There are three ongoing edoxaban pediatric clinical trials, one phase 1 study (NCT02303431) and two phase 3 trials (NCT02798471 and NCT03395639), but the results are not available yet.
- Direct thrombin inhibitors. Dabigatran is the only orally available direct thrombin inhibitor, and it was the first DOAC approved for children. This approval was based on the results from two open-label clinical trials (NCT01895777 and NCT02197416), and a subgroup analysis of children with all types of thrombophilia. These studies showed that dabigatran was as effective and safe as standard of care therapies to treat and prevent blood clots in patients younger than 18 years, including those with thrombophilia.
VTE prevention
Pediatric data on VTE prophylaxis are limited and indications for primary prophylaxis in children without a prior VTE are not well established. The number and nature of risk factors for VTE, whether the risk factors are transient or chronic, and the bleeding risk are important considerations to keep in mind when deciding to start a prophylactic regimen. In general, thromboprophylaxis should be continued only while the risk factors persist.
There are VTE risk-prediction and decision algorithms available, however, these have not been validated in a prospective study. There are guidelines for pediatric trauma patients but they are based on very low-quality evidence. Nevertheless, there are limited data from clinical trials and observational studies that support the efficacy of pharmacologic thromboprophylaxis is hospitalized children with risk factors for VTE, particularly those with prior VTE.
For hospitalized pediatric patients who are acutely ill or recovering from surgery, mechanical methods to reduce the risk of VTE are encouraged, if adequate devices are available, for patients with risk factors for developing VTE. Early mobilization is encouraged whenever possible and it may be sufficient prophylaxis for low-risk patients. The use of prophylactic anticoagulation therapy is limited to patients with multiple risk factors and with a low risk of bleeding complications. A prior history of VTE and known thrombophilia are particularly strong predictors of a VTE event, and the presence of either of these risk factors alone may warrant pharmacologic thromboprophylaxis during an acute hospitalization, provided that there are no contraindications.
There are certain conditions or circumstances that represent a particularly high thrombotic risk for hospitalized children. These are cardiac surgery, severe acute heart failure, Kawasaki disease, acute severe traumatic injury, COVID-19-related multisy

stem inflammatory syndrome, asparaginase therapy for acute lymphoblastic leukemia, acute flare of inflammatory bowel disease, and nephrotic syndrome. While a discussion on pharmacologic thromboprophylaxis for each of these conditions is beyond the scope of this activity, it is important that health care professionals are aware of the potential need for this type of intervention.
For patients who have a considerable long-lasting risk of VTE, the use of long-term antithrombotic therapy may be warranted. These include patients receiving long-term home total parenteral nutrition, patients undergoing hemodialysis via an arteriovenous fistula or central venous access device, some patients with congenital and infantile nephrotic syndrome, and children with certain forms of cardiac disease. Long-term anticoagulant thromboprophylaxis may also be indicated for patients with a prior episode of VTE as well as either a prior history of provoked VTE with ongoing or recurrent risk factors, or recurrent unproved VTE.
A long-term complication of VTE is the development of PTS, which arises because of chronic venous occlusion or valvular disruption and leads to venous hypertension. Minimizing the risk of clot formation and encouraging clot resolution reduces the risks of PTS in pediatric patients, and the early institution of anticoagulation therapy is a key step to achieve these goals. Cardiometabolic Health Congress faculty member Sonja D. Bartolome, MD, directs the Chronic Thromboembolic Pulmonary Hypertension program at the University of Texas Southwestern Medical Center, where she works with a multispecialty team to perform curative procedures such as pulmonary thromboendarterecomy (PTE) surgery or balloon pulmonary angioplasty (BPA).
Furthermore, recurrent VTE that limits adequate venous access can become a life-limiting condition in medically complex pediatric patients that are dependent on venous access for life sustaining measures, such as those with congenital heart disease and short bowel syndrome. The use of anticoagulants as secondary prophylaxis can reduce the risk of VTE recurrence in these patients.
Thrombolysis
Anticoagulation alone may not be sufficient to prevent long-term morbidity associated with acute VTE. The use of exogenous agents for more rapid dissolution of the thrombus burden is termed thrombolysis, and the most common thrombolytic agent used in children is recombinant tissue plasminogen activator (rtPA). While thrombolytic therapy is generally reserved for life-, limb-, and organ-threatening events, its use for the management of pediatric VTE is increasing, especially in centers with pediatric interventional radiologists and cardiologists with an expertise in this therapy. However, it is important to note that the American Society of Hematology guidelines recommend against thrombolysis for most patients.
The administration of rtPA can be done systemically or by catheter-directed thrombolysis (CDT), where a low dose infusion is administered through a catheter with the tip situated within the thrombus. While there are no studies comparing the routes of administration, case series suggest that CDT is safer and more efficacious.39
Discussion
Pediatric VTE is a serious medical condition and cases have been increasing over the last two decades, especially for hospitalized children. However, few clinical trials have been done in this population group, which means that most treatment guidelines are extrapolated from results from clinical trials done in adults. Furthermore, most well established anticoagulation agents are particularly difficult to use in children as they cannot be given orally. In the last year, two new oral anticoagulation agents were approved for treating and preventing pediatric VTE, and a clinical trial showed that the treatment duration can be shortened substantially for certain patients. This activity will review the risk factors and clinical manifestations of pediatric VTE, discuss prevention and treatment guidelines, and introduce the newly approved pediatric anticoagulation agents.
Finally, while children are less susceptible to severe cases of coronavirus disease 2019 (COVID-19), acute COVID-19 infections are associated with hypercoagulability and thromboembolic complications. Therefore, it is important that healthcare professionals know how to recognize and treat pediatric VTE in the context of the ongoing COVID-19 pandemic.