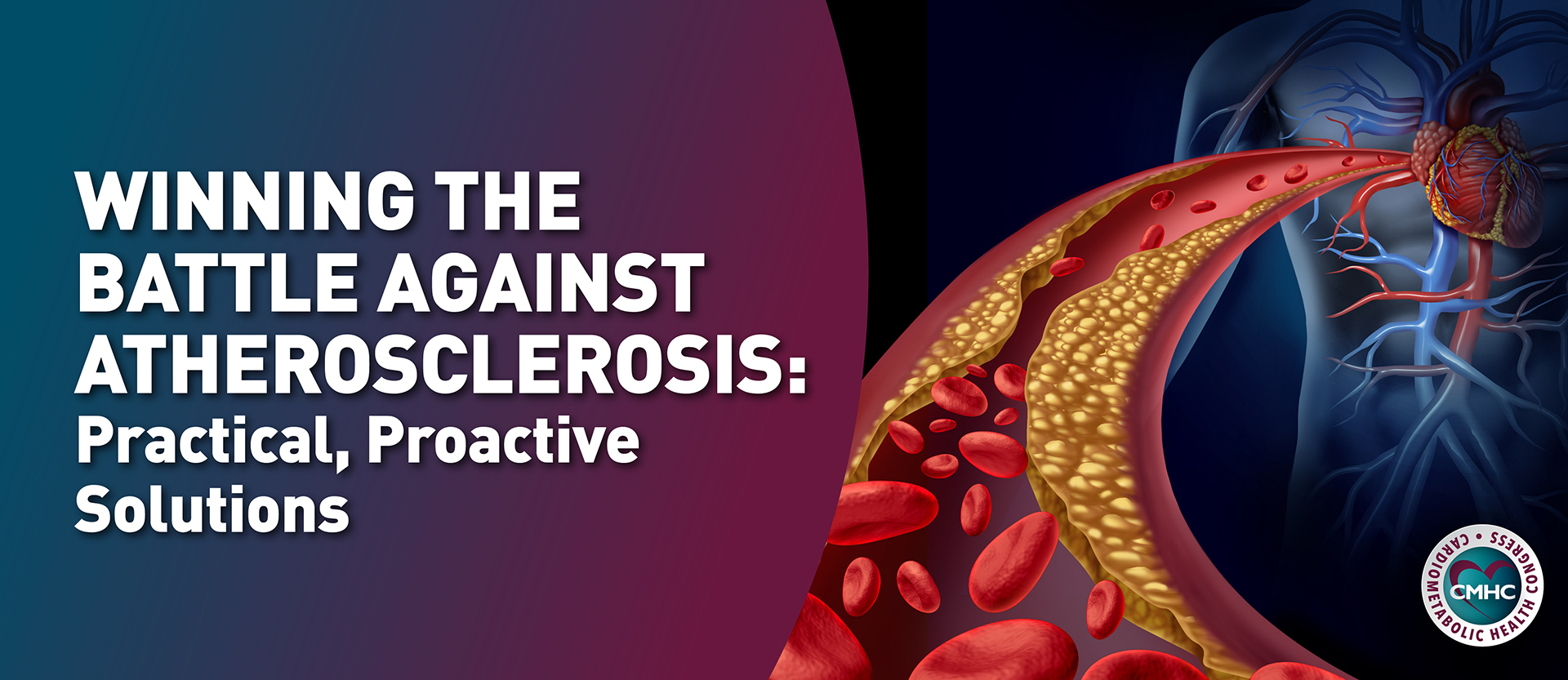
The experts at Cardiometabolic Health Congress (CMHC) have selected the TOP 10 developments, approvals, news, and findings from 2022 that impact the field of cardiorenal metabolic health: the intersection of diseases affecting heart, kidney, endocrine and overall health.
10. Finerenone Improves Outcomes in CVD and CKD
“Recent findings from the FIDELITY-HF trial support the use of finerenone and highlight the importance of routine screening for renal function in primary care to identify kidney decline early in patients with diabetes.”
“GRADE showed that any of these four medications, representing four major glucose-lowering drug classes in use at the time of trial design and today, can be effective for the management of hyperglycemia in type 2 diabetes.”
8. AHA Update: Life’s Simple 7 Now Life’s Essential 8 After Addition of Sleep Metric
“Drawing on data from the 2017 American Heart Association (AHA) Presidential Advisory and a 2021 Scientific Statement on the mind-heart-body connection, the updated checklist includes another vital metric of cardiovascular health: sleep.”
7. Reno-Protective Effects of SGLT2 Inhibitors
“As demonstrated by the EMPA-KIDNEY trial, SGLT2 inhibitors have not only transformed diabetes care, but are also having a major impact on the care of patients with heart failure and chronic kidney disease.”
6. Tirzepatide for Treating Obesity
“Compared with placebo, patients receiving the highest dose of tirzepatide (15 mg weekly) achieved a 17.8% weight reduction, with 57% of patients in this arm achieving a weight loss of 20% at 72 weeks. These results are comparable to those reported in the bariatric surgery literature.”
5. FDA Approves First Drug To Delay Onset of Type 1 Diabetes
“Today’s approval of the first-in-class teplizumab adds an important new treatment option for certain at-risk patients. The drug’s potential to delay clin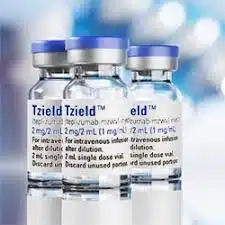 ical diagnosis of type 1 diabetes may provide patients withmonths to years without the burdens of disease.”
ical diagnosis of type 1 diabetes may provide patients withmonths to years without the burdens of disease.”
4. Updated 2023 ADA Standards of Care
“The American Diabetes Association’s updated standards for 2023 include lower thresholds for cholesterol and blood pressure, new  confidence in recommending certain classes of drugs for renal and cardiovascular protection, and a call for physicians to lean on the entire spectrum of health providers in overcoming barriers to effective diabetes care.”
confidence in recommending certain classes of drugs for renal and cardiovascular protection, and a call for physicians to lean on the entire spectrum of health providers in overcoming barriers to effective diabetes care.”
3. CLEAR Cardiovascular Outcomes Trial Results
“Esperion announced that the CLEAR Outcomes study met its primary endpoint, demonstrating statistically significant risk reduction in MACE-4 in patients treated with 180 mg per day of bempedoic acid (Nexletol) compared to placebo.”
2. FDA Approves Insulin Biosimilar Rezvoglar
“The 2022 approval of Rezvoglar as another interchangeable biosimilar insulin option is a welcomed stride toward insulin affordability and accessibility, which may improve treatment adherence and outcomes for patients with diabetes.”
1. 2022’s FDA-Approved Cardiometabolic Drugs and Devices
 “Although the U.S. Food and Drug Administration (FDA) approved a record-low number of drugs and devices in 2022, many of those were in the realm of cardiorenal metabolic health.”
“Although the U.S. Food and Drug Administration (FDA) approved a record-low number of drugs and devices in 2022, many of those were in the realm of cardiorenal metabolic health.”