Although the U.S. Food and Drug Administration (FDA) approved a record-low number of drugs and devices from January through September 2022, many of those were in the realm of cardiorenal metabolic health.
January 2022
- Daridorexant. In the first major move of 2022, the FDA approved daridorexant for the treatment of adults with insomnia on Jan. 7. The approval resulted from two successful phase III trials in which the drug demonstrated significant improvement in sleep onset, sleep maintenance, and patient-reported sleep time. Sleep is a pillar of cardiometabolic health; hypertension and diabetes risk increase in individuals with sleep disorders and short sleep duration, particularly in younger patients with obesity. Sold under the brand name QUVIVIQ™ by Swiss biotech Idorsia, daridorexant is taken as an oral tablet once per night in either 25 or 50 mg doses.
- Faricimab-svoa. On Jan. 31, the FDA approved f
 aricimab-svoa, the first injectable bispecific antibody for the eye, to treat age-related macular degeneration (nAMD) and diabetic macularedema (DME). Sold by Roche as Vabysmo, the drug’s double-pathway mechanism of action treats retinal conditions that threaten vision, including DME, which is damage to the blood vessels in the retina that results from high blood sugar over time in diabetic patients.
aricimab-svoa, the first injectable bispecific antibody for the eye, to treat age-related macular degeneration (nAMD) and diabetic macularedema (DME). Sold by Roche as Vabysmo, the drug’s double-pathway mechanism of action treats retinal conditions that threaten vision, including DME, which is damage to the blood vessels in the retina that results from high blood sugar over time in diabetic patients.
February 2022
In a very busy month for cardiometabolic drug and device approvals, February of 2022 also saw a relevant drug’s approval date pushed back due to COVID-19-related delays.
- Eversense E3 Continuous Glucose Monitoring System. Senseonics gained approval on Feb. 10 for a new version of its continuous glucose monitoring (CGM) system to be worn for up to 180 days. Previous versions of the sensor were approved by the FDA for only 90 days of wear. The Eversense E3 CGM provides blood glucose readings every five minutes for people with diabetes. The system consists of “an implantable fluorescence-based sensor, a transmitter, and a mobile app for displaying glucose values, trends, and alerts on the patient’s compatible mobile device.”
- CardioMEMS HF System. Abbott (formerly St. Jude Medical) gained expanded approval on Feb. 18 for its CardioMEMS HF System, a wireless device that measures and monitors pulmonary artery (PA) pressure and heart rate for patients with heart failure (HF). The system, consisting of an implantable PA sensor, delivery syst
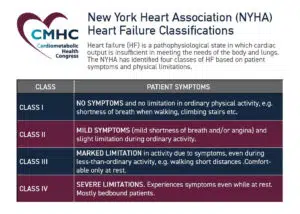 em, and patient electronics system, was initially approved in 2014 for patients with New York Heart Association (NYHA) Class III HFwith a HF-related hospitalization in the year prior. The new indication, supported by clinical data from the GUIDE-HF trial, allows the device to be used by people with earlier-stage HF (NYHA Class II) and for patients who undergo a blood test showing elevated levels of natriuretic peptides, which indicate worsening HF.
em, and patient electronics system, was initially approved in 2014 for patients with New York Heart Association (NYHA) Class III HFwith a HF-related hospitalization in the year prior. The new indication, supported by clinical data from the GUIDE-HF trial, allows the device to be used by people with earlier-stage HF (NYHA Class II) and for patients who undergo a blood test showing elevated levels of natriuretic peptides, which indicate worsening HF. - Empagliflozin. On Feb. 24, the FDA expanded its approval of empagliflozin (Jardiance®) for use in adults with HF, regardless of ejection fraction, to reduce the risk of cardiovascular death and HF hospitalization. The new indication is updated from the Boehringer Ingelheim and Eli Lilly and Company drug’s Aug. 2021 label to reduce the risk of cardiovascular death and HF hospitalization in adults with HF with reduced ejection fraction (HFrEF), as well as the original 2014 approval, which was only for glucose control in adults with type 2 diabetes.
March 2022
- Luspatercept-aamt DELAYED. On March 28, the FDA announced that it was delaying its decision on Bristol Myers Squibb’s luspatercept-aamt (Reblozyl) for the treatment of patients with non transfusion-dependent beta thalassemia anemia until June 27. The agency asked for additional information before it made a decision on the agent, which was already approved for use in patients with “anemia with beta thalassemia who need regular blood transfusions and certain patients with very low-to-intermediate risk myelodysplastic syndrome who have anemia that did not respond to an erythropoiesis stimulating agent.”
- Aveir™ Leadless Pacemaker System. On March 31, based on a clinical trial with 526 patients showing the device to be safe and effective for single cha
 mber pacing, the FDA approved Abbott’s Aveir Leadless Pacemaker for use in “patients with slow or irregular heart rhythms who may benefit from a pacemaker system that works in one chamber of the heart (single chamber pacemaker system). This system may also be used if a traditional pacing system with leads is hard to place.”
mber pacing, the FDA approved Abbott’s Aveir Leadless Pacemaker for use in “patients with slow or irregular heart rhythms who may benefit from a pacemaker system that works in one chamber of the heart (single chamber pacemaker system). This system may also be used if a traditional pacing system with leads is hard to place.”
April 2022
- Thoraflex Hybrid. Scotland’s Vascutek won approval on April 19 for its device to repair a weakened and bulging section (aneurysm) or a tear in the lining (dissection) of the aorta behind the heart (aortic arch). The Th
 oraflex Hybrid includes a “polyester graft section that reinforces a weakened section of the blood vessel, a connected-stented section (nitinol wire frame on polyester graft material) that holds the artery open, and a delivery catheter that is used to place the device.” It is used during open-heart surgery to repair or replace damaged or sections of the aorta.
oraflex Hybrid includes a “polyester graft section that reinforces a weakened section of the blood vessel, a connected-stented section (nitinol wire frame on polyester graft material) that holds the artery open, and a delivery catheter that is used to place the device.” It is used during open-heart surgery to repair or replace damaged or sections of the aorta. - Mavacamten.On April 28, the FDA approved Mavacamten (Camzyos™) 2.5mg, 5mg, 10mg, and 15mg capsules for the treatment of adults with symptomatic NYH
 A Class II and III obstructive hypertrophic cardiomyopathy (HCM) to improve functional capacity and symptoms. The drug is the first and only FDA-approved allosteric and reversible inhibitor selective for cardiac myosin and “builds on decades of cardiovascular leadership and reflects our steadfast commitment to people impacted by cardiovascular disease,” according to Samit Hirawat, MD, executive vice president and chief medical officer, Global Drug Development, Bristol Myers Squibb.
A Class II and III obstructive hypertrophic cardiomyopathy (HCM) to improve functional capacity and symptoms. The drug is the first and only FDA-approved allosteric and reversible inhibitor selective for cardiac myosin and “builds on decades of cardiovascular leadership and reflects our steadfast commitment to people impacted by cardiovascular disease,” according to Samit Hirawat, MD, executive vice president and chief medical officer, Global Drug Development, Bristol Myers Squibb. - ENROUTE Transcarotid Stent System. Silk Road Medical’s self-expanding stent and a delivery catheter system was previously approved to re-open narrowed parts of the carotid arteries in patients at high risk for complications from surgery. On April 28, the FDA expanded the approval to include its use in patients with a normal risk of complications from surgery for this condition. This expansion was based on “real-world data collected from a patient registry showed that 2,962 patients who received the ENROUTE Transcarotid Stent System and ENROUTE NPS together did no worse than 8,886 similar patients who received the surgical treatment instead.”
May 2022
- Tirzepatide. In perhaps the biggest news for providers who treat patients with diabetes, the FDA approved a novel, dual-targeted treatment for type 2 diabetes on May 13. In clinical stu
 dies, tirzepatide (Mounjaro) was shown to improve blood sugar control in adults with type 2 diabetes better than other therapies with which it was compared. Eli Lilly said Mounjaro “is a first-in-class medicine that activates both the GLP-1 and GIP receptors, which leads to improved blood sugar control.” The drug is administered as a once-weekly adjusted-dose subcutaneous injection to meet blood sugar goals.
dies, tirzepatide (Mounjaro) was shown to improve blood sugar control in adults with type 2 diabetes better than other therapies with which it was compared. Eli Lilly said Mounjaro “is a first-in-class medicine that activates both the GLP-1 and GIP receptors, which leads to improved blood sugar control.” The drug is administered as a once-weekly adjusted-dose subcutaneous injection to meet blood sugar goals. - Treprostinil. On May 24, United Therapeutics received FDA approval for its therapeutic treprostinil inhalation powder (Tyvaso DPI), marking the first approval of a dry powder inhaler for the treatment of pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease (PH-ILD). “Tyvaso DPI is one of the easiest ways for patients to administer a prostacyclin, delivering the proven efficacy of treprostinil through a small inhaler that fits in the palm of the patient’s hand,” said Michael Benkowitz, President and Chief Operating Officer of United Therapeutics. “We look forward to launching this exciting new product, and the opportunity to introduce treprostinil to more patients with PAH and PH-ILD.”
June 2022
-
Luspatercept-aamt WITHDRAWL. On June 3, the maker of luspatercept-aamt (Reblozyl) announced that it was withdrawing its bid for supplemental approval of the drug to treat anemia in adults with non transfusion-dependant beta thalassemia. “While we will not pursue this indication in the U.S., we’re continuing to evaluate Reblozyl® in a broad clinical development program to bring this important therapeutic option to more patients living with the burden of anemia,” said Noah Berkowitz, M.D., Ph.D., senior vice president, Hematology Development, Bristol Myers Squibb.
-
FreedomFlow Peripheral Guidewire. Cardio Flow announced on June 23 that it received the FDA’s 510(k) clearance for its guidewire to treat peripheral artery disease. The FreedomFlow Peripheral Guidewire, which has a stainless-steel core with a silicone-coated distal-spring coil, was developed to support other diagnostic and therapeutic devices that treat plaque blockages in arteries both above and below the knee.
Nothing to report for July or August 2022
September 2022
- Organ Care System (OCS) Heart System. The TransMedics, Inc. OCS Heart System was approved on Sept. 3 for use by “trained medical professionals to transport donor-after-brain-death hearts when the cold storage method is unsuitable.” The system supplies donor hearts with oxygen and nutrients, includes a portable warming enclosure, and measures and displays parameters such as temperature and pressure.
- Tenapanor. The battle between tenapanor’s maker Ardelyx and the FDA began back in July 2021. In Phase III trials, the NHE3 inhibitor met all primary and secondary endpoints for efficacy, safety, and tolerance, but the FDA denied approval, stating that although clinical data provided evidence of efficacy, the breadth of efficacy was small and of unclear clinical significance. Another denial was confirmed in Feb.2022, but finally, on Sept. 12, 2022, Ardelyx announced that “
 Isbrela® (tenapanor), a 50 mg, twice-daily oral pill for the treatment of irritable bowel syndrome with constipation (IBS-C) in adults” had been FDA-approved in a hard-fought development. Ardelyx says the drug is “a minimally absorbed small molecule that acts locally in the gastrointestinal (GI) tract to inhibit the sodium-hydrogen exchanger NHE3, resulting in an increase in bowel movements and a decrease in abdominal pain for patients with IBS-C.”
Isbrela® (tenapanor), a 50 mg, twice-daily oral pill for the treatment of irritable bowel syndrome with constipation (IBS-C) in adults” had been FDA-approved in a hard-fought development. Ardelyx says the drug is “a minimally absorbed small molecule that acts locally in the gastrointestinal (GI) tract to inhibit the sodium-hydrogen exchanger NHE3, resulting in an increase in bowel movements and a decrease in abdominal pain for patients with IBS-C.” - Terlipressin. On Sept. 14, the FDA named Mallinckrodt’s terlipression (Terlivaz) the first-ever drug to improve kidney function in adults with hepatorenal syndrome (HRS) with rapid reduction in kidney function. “The phase 3 CONFIRM trial evaluated the safety and efficacy of terlipression for HRS type 1 patients in the United States and Canada. The largest prospective study using terlipressin in HRS involving rapid reduction in kidney function, the trial met the primary endpoint of renal function improvement without using dialysis, and short-term survival.”
October 2022
- Furosemide. On Oct. 7, scPharmaceuticals, Inc. gained the FDA’s approval of furosemide (Furoscix), a novel diuretic indicated for the treatment of congestion due to fluid overload in adults with NYHA HF Class II and III. Th
 e subcutaneous formulation of the diuretic is delivered at-home via a wearable, pre-programmed on-body drug delivery system. “Congestion due to worsening heart failure is one of the most common causes of hospital admissions in patients over 65, and today’s approval of Furoscix represents an important treatment advancement for the over seven million heart failure patients in the U.S. that will be able to self-administer IV equivalent diuresis at home,” said John Tucker, president and chief executive officer of scPharmaceuticals.
e subcutaneous formulation of the diuretic is delivered at-home via a wearable, pre-programmed on-body drug delivery system. “Congestion due to worsening heart failure is one of the most common causes of hospital admissions in patients over 65, and today’s approval of Furoscix represents an important treatment advancement for the over seven million heart failure patients in the U.S. that will be able to self-administer IV equivalent diuresis at home,” said John Tucker, president and chief executive officer of scPharmaceuticals.
Expected decisions for the remainder of 2022
- Omecamtiv mecarbil. This novel therapy is a cardiac-specific myosin activator being studied for a potential role in the treatment of left ventricular systolic heart failure. Its application is supported by findings from the phase 3 GALACTIC-HF trial, a composite HF event or cardiovascular event-driven outcome assessment that showed patients with HFrEF had insignificantly improved risk reduction with omecamtiv mecarbil versus placebo. However, patients with ≤28% ejection fraction were observed to report greater composite risk reduction with the therapy versus placebo. The FDA has a projected target action date of Nov. 30, 2022. “This is an exciting milestone and important next step towards the commercial launch of omecamtiv mecarbil,” said Robert I. Blum, Cytokinetics’ president and chief executive officer. “More than two million people in the U.S. with HFrEF have signs and symptoms of worsening heart failure despite standard of care therapy, pointing to a clear unmet medical need for more treatment options. We look forward to engaging with the FDA to bring this potential new medicine to patients later this year.”
- Aprocitentan. This investigational dual endothelin receptor antagonist potently inhibits the binding of
 ET-1 to ETA and ETB receptors; it has a long half-life, a low potential for drug-drug interaction and a mechanism of action that is ideally suited for the pathophysiology of difficult-to-treat forms of hypertension. ThePRECISION trial investigated the antihypertensive effect of the new drug in combination with other antihypertensive drugs in refractory hypertension. The results of the trial showed that the antihypertensive effect of Aprocitentan lasted longer. Idorsia Pharmaceuticals and Janssen Biotech have joint development rights over aprocitentan.
ET-1 to ETA and ETB receptors; it has a long half-life, a low potential for drug-drug interaction and a mechanism of action that is ideally suited for the pathophysiology of difficult-to-treat forms of hypertension. ThePRECISION trial investigated the antihypertensive effect of the new drug in combination with other antihypertensive drugs in refractory hypertension. The results of the trial showed that the antihypertensive effect of Aprocitentan lasted longer. Idorsia Pharmaceuticals and Janssen Biotech have joint development rights over aprocitentan. - DefenCath. Already available in Europe and other territories under the brand name Neutrolin, CorMedix’s Defencath™ is a “proprietary formulation of taurolidine 1.35%, citrate 3.5% and heparin 1000 units/mL that is currently being investigated for use as a catheter lock solution, with the aim of reducing the risk of infections from in-dwelling catheters. Taurolidine, the key compound in Defencath™, is an amino acid derivative with in-vitro studies indicating broad antimicrobial activity against gram-positive and gram-negative bacteria, including antibiotic resistant strains, as well as mycobacteria and clinically relevant fungi including Aspergillus.” This potential approval will affect patients with renal failure who are receiving chronic hemodialysis via a central venous catheter.


















